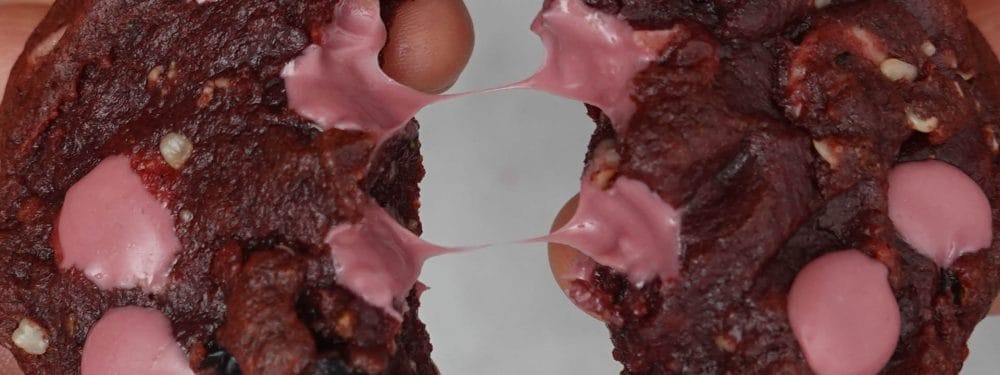
The ice is hot
Big dreams have always driven, motivated and inspired humanity to achieve the unimagined and to enrich and change the world we live in. Flying is such a dream. Another great dream, at least in the culinary world, is hot ice cream.
However, we are not so much hanging on to molecular dreams of the 1990s, our intention has a childish reason. Yes, we like to play and experiment but the reason is not so childish after all. It is about a desire to have children. A little boy from the Food Lab circle of friends dreams of hot ice cream. We took a closer look at why hot ice cream is still not available as a separate variety at every ice cream parlor.
Hot, hotter, hot ice cream
Ice cream classically consists of milk or cream, sugar and egg as well as flavoring ingredients such as vanilla. The interaction of the ingredients and their technical processing provide the delicate melting. Frozen cream would be very hard. Sugar helps to obtain a softer frozen mass. Cream bound with egg yolk increases creaminess, and constant stirring during freezing provides finer ice crystals for a smoother mouthfeel. Liquid nitrogen freezes so quickly at about – 200°C that very small crystals are also formed as a result.
Only how does that translate to hot ice?
So what could hot ice be like? Like a thick bechamel or some kind of marshmallow paste? A little research leads us to different varieties of hot ice cream.
- A rather simple variant plays with the perception of the trigeminal nerve. For this purpose, ice cream or sorbet is made with chilies as an irritant ingredient (since the effect is triggered by the fat-soluble capsaicin, the effect is more intense with water-based ice cream). The pain caused by the capsaicin irritates the perception, the ice cream is really “hot” so spicy. Capsaicin activates the same pain receptors that are activated by heat, so we feel heat despite being cold.
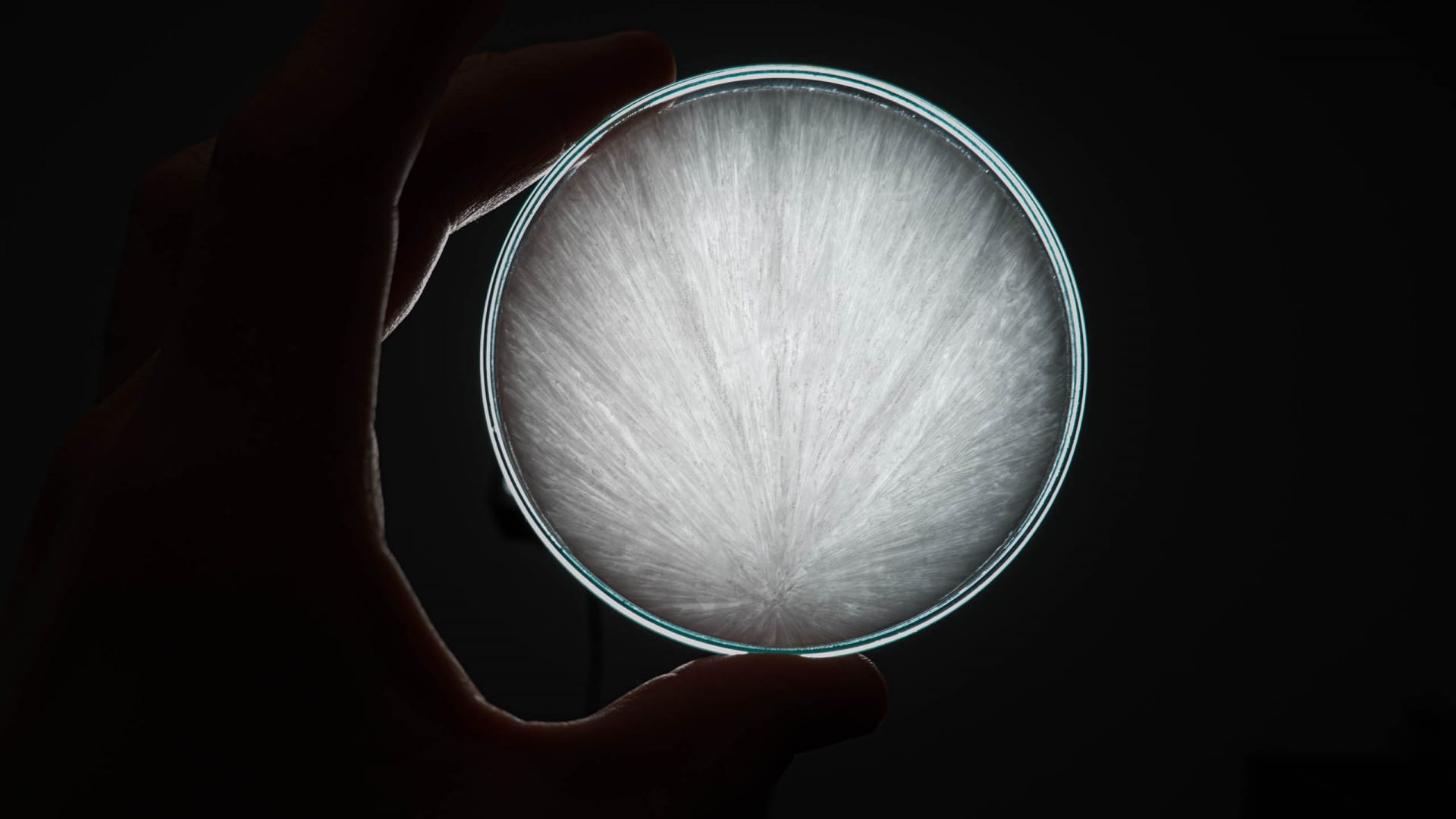
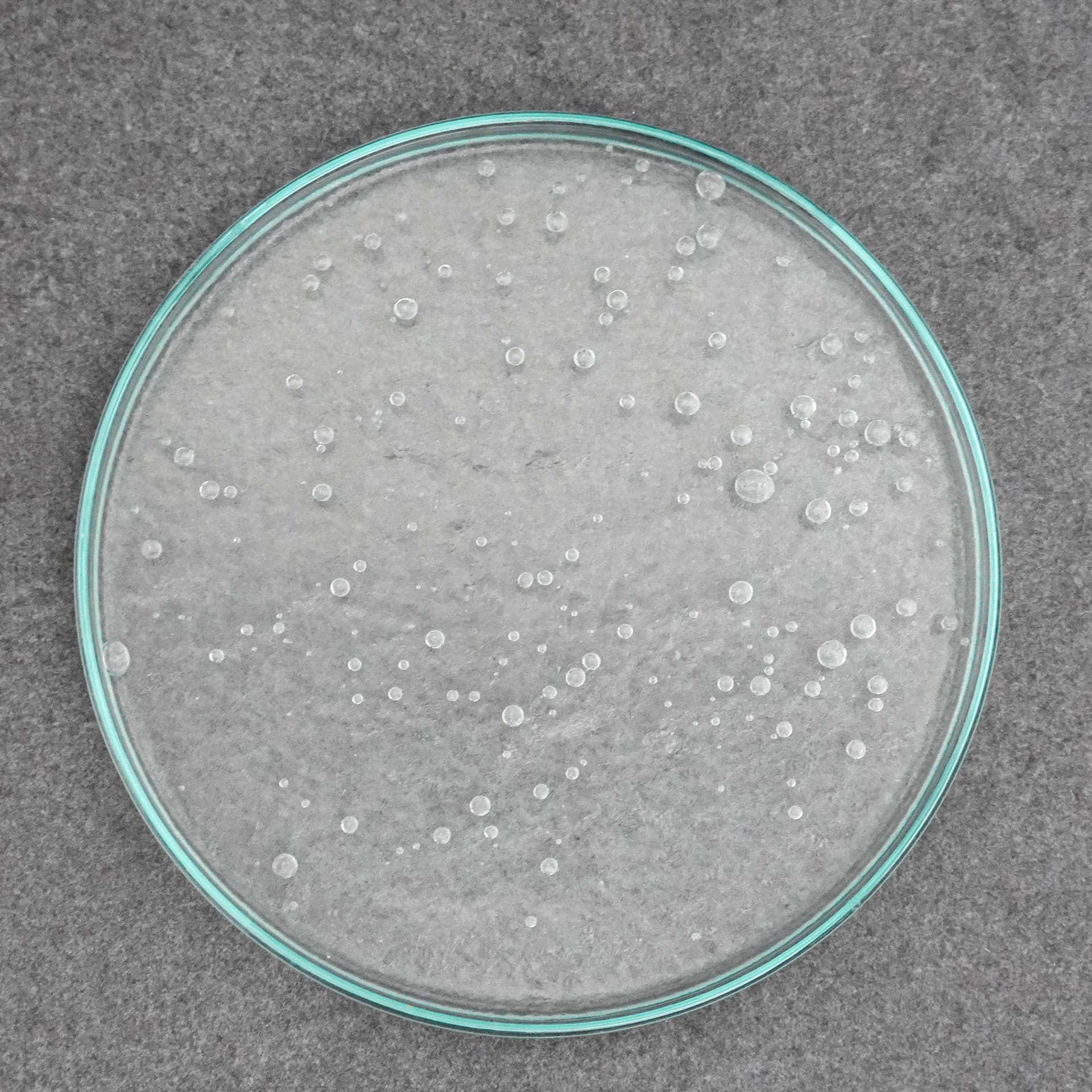
- A second variant is perhaps more familiar from school lessons. For this, sodium bicarbonate is stirred into vinegar and the whole thing is brought to the boil for about 20 minutes over medium heat. Sodium acetate is formed from this. Once the liquid has boiled away, we remove the pot from the heat and observe a slight crystallization. We leave the crystals to cool for 30 minutes and then add a little water. The crystals dissolve again in the water. Normally, the liquid remains clear until it is tapped with a finger. This effect is known from pocket warmers, which work exactly on this principle. A metal plate is bent there. Unfortunately, our vinegar-soda mixture did not show the desired effect after several attempts, but we could still observe a very nice crystallization in the Petri dishes. Well, we are no longer in school lessons and we also want to devote ourselves to culinary experiments with taste. Because even if it works, the ice cream is warm and looks good, but it’s just based on vinegar and sodium, which unfortunately isn’t one of our favorites.
- Variants that use methyl cellulose appear to be more promising. Basically, the marshmallow / bechamel idea is not totally absurd, because methylcellulose is a way to strongly bind warm liquids.
Paste for more mouthfeel
Methyl cellulose binds liquids and is a component of wallpaper paste. Sounds little culinary and, like cellulose (plant cell walls), is indigestible and neither allergenic nor toxic. It is approved as E 461 as a food additive and is used as an emulsifier, stabilizer or thickener whether in creams, mayonnaises or even normal ice cream. For us it is perfect because it gels when heated and loses the gelling property when cooled, reversing properties of ice. This great product, by the way, has made legendary movie scenes possible and is reportedly used as a replacement product in other film industries.
Once popped…
To play with the idea of hot/cold in terms of taste we want to make a popcorn ice cream. For this we pop corn in the pot, add some sugar for the typical taste and let it caramelize. The whole kitchen smells like popcorn. Now the flavor has to go into the ice cream, we heat popcorn with cream and let it cook quietly for about 20 minutes over medium heat. Thus, the flavor and aromas from the caramelized corn pass into the cream. We let the cream cool completely and then strain it through a fine sieve. With this base we can now prepare the ice cream mass. We add milk, brown sugar, water and methyl cellulose. We mix the mass vigorously. During mixing, the properties of methyl cellulose can be observed. The consistency seems creamy almost slightly slimy. Methyl cellulose needs a certain time to develop its effect properly. To do this, we leave the ice cream mixture in the refrigerator for 24 hours. Now we will see whether the children’s dream remains a dream or whether we can call ourselves dream fulfillers from now on.

For the “ice cream”, we boil water and use an ice cream scoop to put balls into the hot water. The ball immediately forms a skin and floats on the water surface. We turn them a few times and then we can taste. Unfortunately, during the taste test we realize that the dream will remain a dream .
The popcorn flavor is present, and the sugar content is balanced. But the consistency is not the consistency a child would want or we would want ice cream to be. A wobbly, rubbery, cut-resistant texture. As soon as we take the balls out of the hot water, they easily collapse and pull a skin. Some dreams must remain dreams – but we continue to experiment and thus live our dreams!